The Power of DHE
D.H.E. 45®, an established migraine and cluster headache therapy, has over 70 years of clinical use1,2

DHE has the potential for a long duration of effect, so it’s usually quite long and associated with low recurrence.


MECHANISM OF ACTION
| Serotonergic (5‑HT) | Noradrenergic | Dopaminergic | CGRP Receptor | |
|---|---|---|---|---|
| D.H.E. 45®2 | 1A, 1Dα, 1Dβ, 2A, 2C | α2A, α2B, α1 | 2L, 3 | |
| Triptans3 | 1B, 1D, 1F | |||
| Ditans4 | 1F | |||
| Gepants/Monoclonal Antibodies5 | CGRP receptor |
DHE can be effective at any point during a migraine or cluster headache attack.6
In addition, DHE†:
- Can reverse central sensitization exhibited in migraine attacks1,7
- Is proven to provide sustained migraine headache relief and reduce headache recurrence8
- Is often an effective treatment for patients with allodynia1
Clinical studies of DHE have shown that it is effective in treating patients experiencing‡,§:
- Acute migraine episodes with or without aura6
- Status migrainosus9,10
- Migraine recurrence1
- Rapid-onset migraine1
- Cluster headache attack6
- Migraine upon awakening11
- Triptan and gepant non-responders1,9,10
- Medication overuse headache9,10
§Bioequivalence demonstrated in a randomized, open-label, crossover bioavailability study in 27 healthy, fasting adults.12
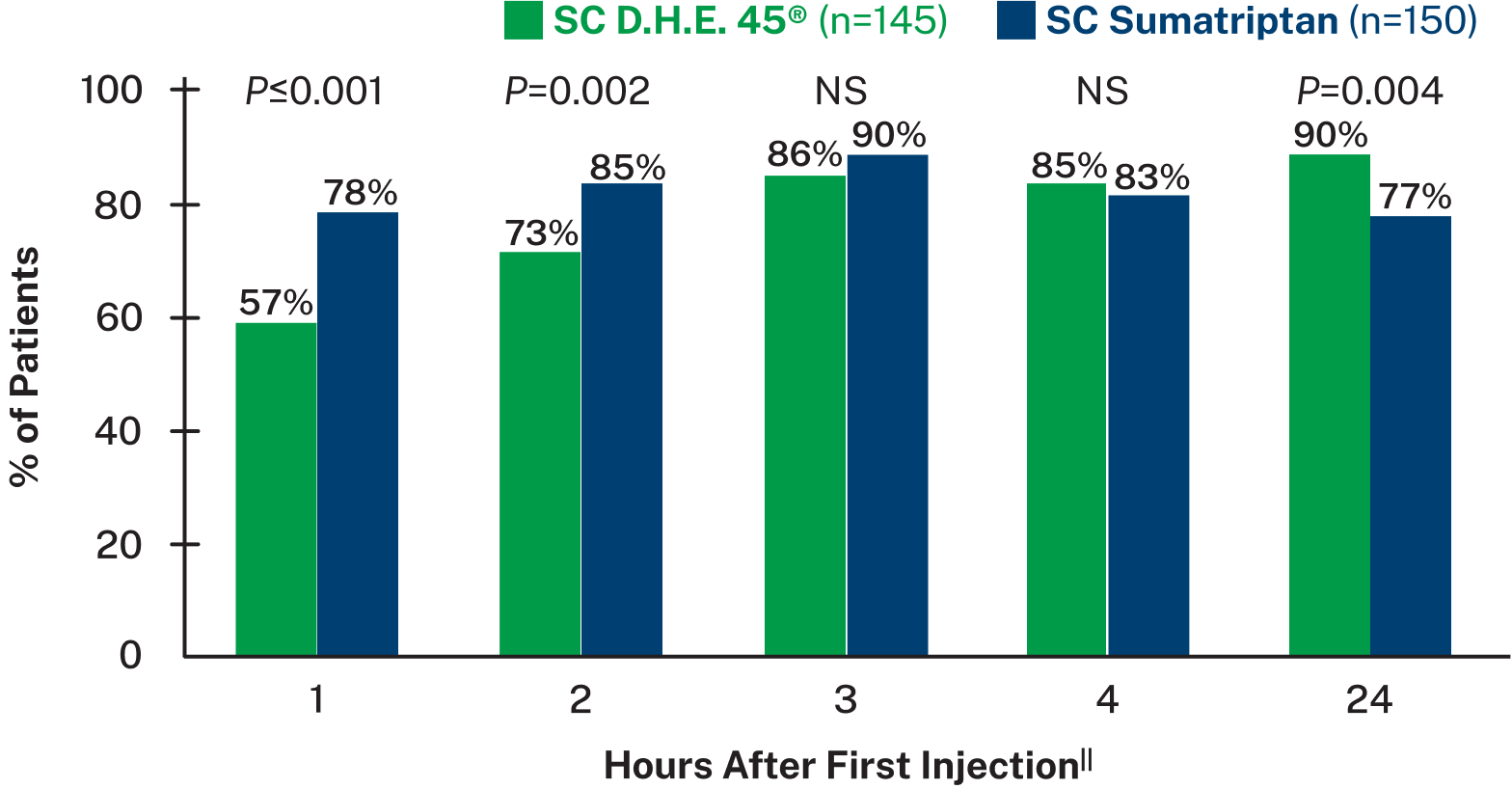
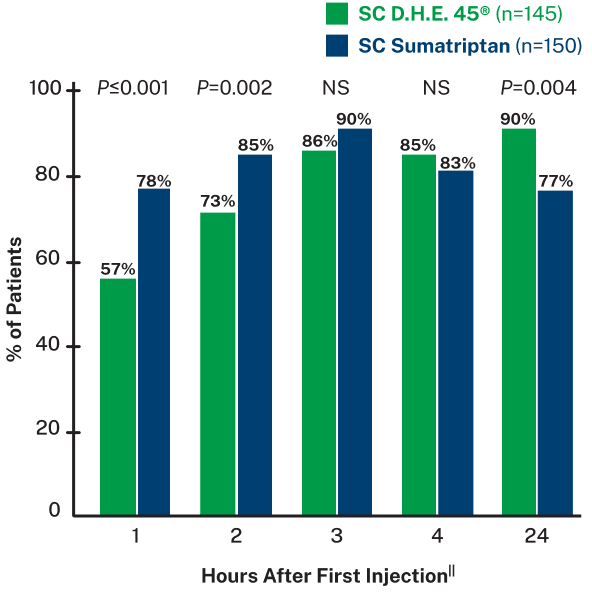
Subcutaneous D.H.E. 45® is effective in sustaining pain relief for migraine8
PATIENTS WITH PAIN RELIEF BY TREATMENT GROUP8,||
Brekiya autoinjector contains DHE, the same active ingredient as D.H.E. 45®.6,8
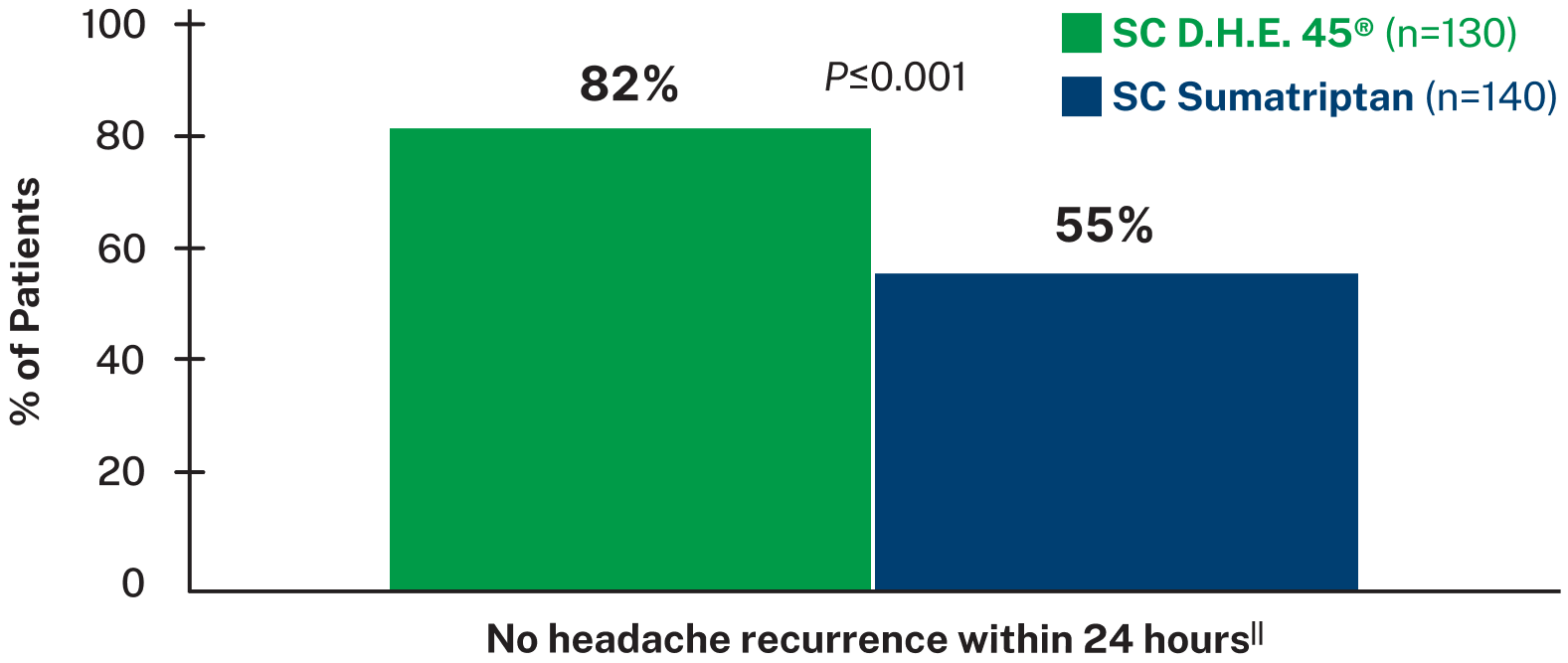
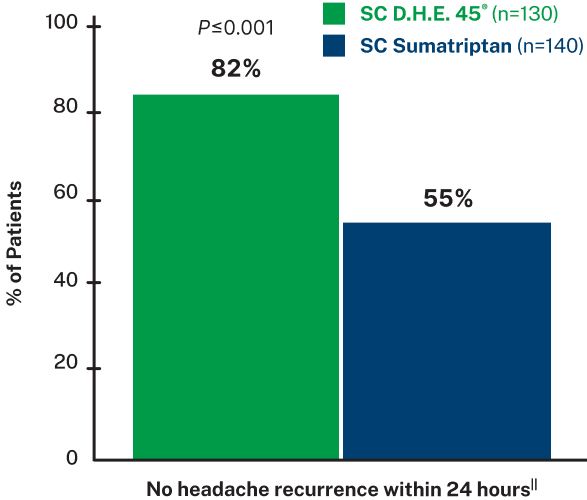
Of patients who reported headache relief, 82% in the SC D.H.E. 45® group and 70% in the SC sumatriptan group reported no pain at all 24 hours post-dose8
Proven to reduce migraine recurrence8
SIGNIFICANTLY MORE D.H.E. 45® PATIENTS HAD NO HEADACHE RECURRENCE VS. SUMATRIPTAN8,||
Brekiya autoinjector contains DHE, the same active ingredient as D.H.E. 45®.6,8
In a double-blind, randomized trial of SC D.H.E. 45® vs. SC sumatriptan, the D.H.E. 45® group had 2.5 times less headache recurrence within 24 hours compared to the sumatriptan group.8

||Data based on a randomized, double-blind trial of 295 adult patients with migraine with or without aura, experiencing moderate to severe head pain. Patients were given SC injections of either D.H.E. 45® (1 mg) or sumatriptan succinate (6 mg) into their lateral thigh and self-reported results from 0.5-24 hours after dose. Pain was rated on a 4 point scale as none, mild, moderate, or severe. Pain relief was defined as a change from moderate or severe at onset, to mild or none. 30% of patients in the D.H.E. 45® group and 15% of patients in the sumatriptan group received a second injection after 2 hours, with further rescue medication administered 1 hour after re-dose if needed. Main outcome measures were relief of head pain and recurrence of successfully treated headache.8
NS=not significant
Headache recurrence was defined as an increase in pain severity from none or mild at least 2 hours after achieving pain relief.8
Intravenous D.H.E. 45® has proven results in cluster headache13,¶
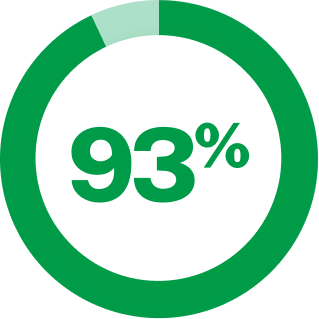
of episodic cluster patients remained completely headache-free 3 months after IV DHE administration13

¶Data based on a study of 54 adult cluster headache patients (episodic and chronic) admitted to hospital on 64 occasions. Patients were given an initial dose of intravenous DHE, and subsequent doses based on response. Patients whose headaches improved were given further doses of IV DHE every 8 hours until they were headache-free for 24 hours. Patients whose headaches persisted were given an additional IV dose 1 hour after the first, followed by additional, larger IV doses every 8 hours until they were headache-free for 24 hours. All patients were then given 2-3 additional IV doses every 12 hours. Patients with nausea were treated with smaller doses. Subsequent breakthrough headaches were treated with self-administered intramuscular injections of DHE. Patients were given metoclopramide throughout the study.13



< PREVIOUS PAGE
NEXT PAGE >
Expand
Collapse
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH STRONG CYP3A4 INHIBITORS
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine with strong CYP3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of Brekiya autoinjector with strong CYP3A4 inhibitors is contraindicated.
Brekiya autoinjector is contraindicated in patients:
- With concomitant use of strong CYP3A4 inhibitors; with peripheral and central vasoconstrictors; and with other 5-HT1 agonists, ergotamine-containing or ergot-type medications within 24 hours.
- With ischemic heart disease or coronary artery vasospasm.
- With uncontrolled hypertension, peripheral arterial diseases, sepsis, following vascular surgery, or severe hepatic or renal impairment.
- Who have hypersensitivity to dihydroergotamine, ergot alkaloids, latex, or any of the ingredients in Brekiya autoinjector.
- Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities: In patients with risk factors predictive of coronary artery disease, consider first dose administration under medical supervision with an electrocardiogram.
- Cerebrovascular Adverse Reactions and Fatalities: Cerebrovascular hemorrhage, subarachnoid hemorrhage, and stroke have been reported; discontinue Brekiya autoinjector if suspected.
- Other Vasospasm Related Adverse Reactions: Brekiya autoinjector may cause vasospasm or elevation in blood pressure. Discontinue if signs or symptoms of vasoconstriction develop.
- Medication Overuse Headache: Detoxification may be necessary.
- Preterm Labor: Advise pregnant women of the risk.
- Fibrotic Complications: Pleural and retroperitoneal fibrosis have been reported following prolonged daily use of dihydroergotamine mesylate. Do not exceed the Brekiya autoinjector dosing guidelines or use for chronic daily administration.
Serious cardiac events (including fatal) that have been reported with dihydroergotamine mesylate injection use include: coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, ventricular fibrillation.
- For subcutaneous injection only.
- Recommended dosage is 1 mg administered subcutaneously as a single 1 mL autoinjector.
- Do not exceed 3 mg (3 doses) in a 24-hour period.
- Do not exceed 6 mg (6 doses) in a week.
- Prior to initiation, a cardiovascular evaluation is recommended.
- Beta Blockers/Nicotine: May potentiate/
provoke vasoconstriction. - Selective Serotonin Reuptake Inhibitors: Weakness, hyperreflexia, and incoordination may occur with coadministration.
- Pregnancy: Based on animal data, may cause fetal harm.
- Lactation: Advise patients not to breastfeed during treatment with Brekiya autoinjector and for 3 days after the last dose.
Brekiya autoinjector is indicated for the acute treatment of migraine with or without aura and the acute treatment of cluster headaches in adults.
Limitations of Use:
Brekiya autoinjector is not indicated for the preventive treatment of migraine or for the management of hemiplegic migraine or migraine with brainstem aura.