About Brekiya autoinjector
The first and only autoinjector to deliver the power of DHE for sustained* headache relief1-4,†
Provides patients the same powerful medication used in hospitals, now in a disposable, ready-to-use, single-dose autoinjector that delivers one dose subcutaneously into the thigh1,5
May be helpful for patients who respond inadequately to oral therapies due to nausea/vomiting, gastroparesis, or delayed dosing5,6
Gives patients the ability to self-administer, with no need for refrigeration, assembly, or priming the device1
*Sustained represents a duration of approximately 24-72 hours.3,4
†Cluster headache results reported using IV administration.4
Brekiya autoinjector achieves peak concentration levels in only 24 minutes2
| Administration | Bioavailability level | Peak Concentration Levels |
|---|---|---|
| DHE Nasal Formulations | 15-59%7,8 | 30-60 mins7,9 |
| Brekiya autoinjector | 100%10 | 24 mins2 |
A subcutaneous administration route offers a high bioavailability along with a prompt Tmax2,10
Brekiya autoinjector is bioequivalent to a subcutaneous D.H.E. 45® injection2,‡
MEAN PLASMA CONCENTRATION
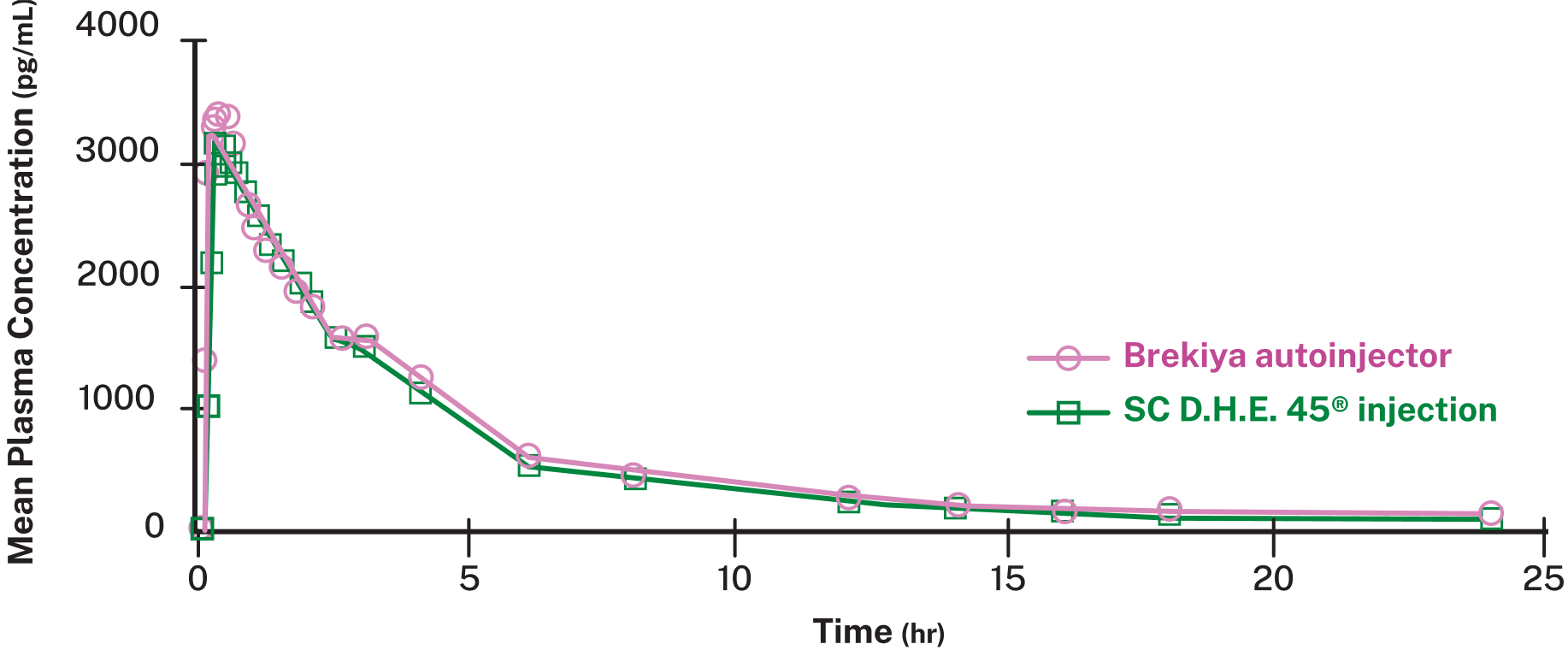
SC=subcutaneous
‡Bioequivalence demonstrated in a randomized, open-label, crossover bioavailability study in 27 healthy, fasting adults.2

It’s going to increase our toolbox and our opportunities for helping patients.


Brekiya autoinjector is ready-to-use, with no need for refrigeration, assembly, or priming the device1
DOSING INFORMATION
- Single-dose, prefilled, disposable device1
- Recommended dosage is 1 mg subcutaneously1
- The dose can be repeated, as needed, at 1 hour intervals to a total dose of 3 mg (3 doses of 1 mg) subcutaneous delivery in a 24 hour period1
- Total weekly dosage should not exceed 6 mg1
- Brekiya autoinjector should not be used for chronic daily administration1

For illustration purposes only. Not actual size. Brekiya autoinjector uses a 27 gauge needle.2
Watch a step-by-step video on how to use Brekiya autoinjector safely and confidently.
When following instructions for use, 100% of patients successfully self administered a dose with the Brekiya autoinjector2,§
NEXT PAGE >
Expand
Collapse
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH STRONG CYP3A4 INHIBITORS
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine with strong CYP3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of Brekiya autoinjector with strong CYP3A4 inhibitors is contraindicated.
Brekiya autoinjector is contraindicated in patients:
- With concomitant use of strong CYP3A4 inhibitors; with peripheral and central vasoconstrictors; and with other 5-HT1 agonists, ergotamine-containing or ergot-type medications within 24 hours.
- With ischemic heart disease or coronary artery vasospasm.
- With uncontrolled hypertension, peripheral arterial diseases, sepsis, following vascular surgery, or severe hepatic or renal impairment.
- Who have hypersensitivity to dihydroergotamine, ergot alkaloids, latex, or any of the ingredients in Brekiya autoinjector.
- Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities: In patients with risk factors predictive of coronary artery disease, consider first dose administration under medical supervision with an electrocardiogram.
- Cerebrovascular Adverse Reactions and Fatalities: Cerebrovascular hemorrhage, subarachnoid hemorrhage, and stroke have been reported; discontinue Brekiya autoinjector if suspected.
- Other Vasospasm Related Adverse Reactions: Brekiya autoinjector may cause vasospasm or elevation in blood pressure. Discontinue if signs or symptoms of vasoconstriction develop.
- Medication Overuse Headache: Detoxification may be necessary.
- Preterm Labor: Advise pregnant women of the risk.
- Fibrotic Complications: Pleural and retroperitoneal fibrosis have been reported following prolonged daily use of dihydroergotamine mesylate. Do not exceed the Brekiya autoinjector dosing guidelines or use for chronic daily administration.
Serious cardiac events (including fatal) that have been reported with dihydroergotamine mesylate injection use include: coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, ventricular fibrillation.
- For subcutaneous injection only.
- Recommended dosage is 1 mg administered subcutaneously as a single 1 mL autoinjector.
- Do not exceed 3 mg (3 doses) in a 24-hour period.
- Do not exceed 6 mg (6 doses) in a week.
- Prior to initiation, a cardiovascular evaluation is recommended.
- Beta Blockers/Nicotine: May potentiate/
provoke vasoconstriction. - Selective Serotonin Reuptake Inhibitors: Weakness, hyperreflexia, and incoordination may occur with coadministration.
- Pregnancy: Based on animal data, may cause fetal harm.
- Lactation: Advise patients not to breastfeed during treatment with Brekiya autoinjector and for 3 days after the last dose.
Brekiya autoinjector is indicated for the acute treatment of migraine with or without aura and the acute treatment of cluster headaches in adults.
Limitations of Use:
Brekiya autoinjector is not indicated for the preventive treatment of migraine or for the management of hemiplegic migraine or migraine with brainstem aura.